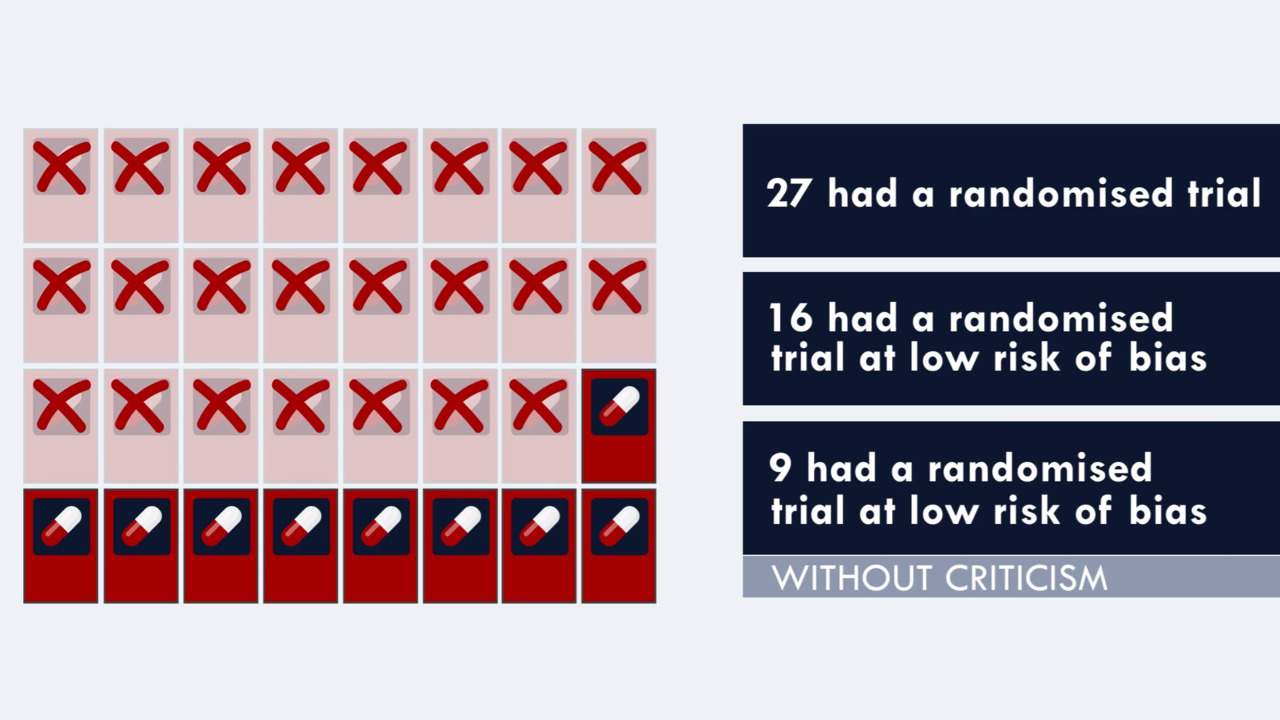
41 open-label trial disadvantages
External and internal validity of open label or double‐blind ... Sep 15, 2011 · Naturally, in open-label trials in anticoagulation there is a risk of a reporting bias of adverse events. Patients may research the new drug and its side-effects in publications and may be influenced in their reporting behaviour of potential side-effects. Furthermore, investigators may be equally susceptible to a reporting bias. External and internal validity of open label or double-blind ... In these trials, open-label or double-blind double-dummy designs are being used to evaluate the efficacy and safety in prevention and treatment of venous thromboembolism or stroke prevention in atrial fibrillation in several thousands of patients.
What is an Open-Label Clinical Trial? - News-Medical.net Web31 mar. 2022 · Open-label trials are insufficient for providing data on these reactions. Open-label trials can increase the confidence about incidence rates, but as they are …

Open-label trial disadvantages
Open-label trial - Wikipedia WebOpen-label trials may be appropriate for comparing two similar treatments to determine which is most effective, such as a comparison of different prescription anticoagulants, or possible relief from symptoms of some disorders when a placebo is given. An open-label … What is an Open-Label Clinical Trial? - News-Medical.net Clinical trials are vital for the design and development of safe drugs, treatments, and medical interventions and bringing them to market. Often, the idea for a clinical trial starts in the laboratory. Researchers will test new drugs and treatments in animal models, with the most promising being considered for clinical trials. There are four phases... Open-label trial - Wikipedia Open-label trials may be appropriate for comparing two similar treatments to determine which is most effective, such as a comparison of different prescription anticoagulants, [4] or possible relief from symptoms of some disorders when a placebo is given. [5] An open-label trial may still be randomized.
Open-label trial disadvantages. (PDF) What is an open label trial? - ResearchGate May 23, 2014 · The small sample size and the dropout rate of 40% limit the generalisability of the results, as well as increase the risk of type 2 errors (Sullivan & Feinn, 2012). Another limitation is linked to... Prospective, Multi-center, Open-label, Single-arm Registration Trial of ... None (Open Label) Primary Purpose: Treatment: Official Title: PROSPECTIVE, MULTI-CENTER, OPEN-LABEL, SINGLE-ARM REGISTRATION TRIAL OF THE TUBRIDGE FOR THE TREATMENT OF WIDE-NECKED SMALL AND MEDIUM-SIZED INTRACRANIAL ANEURYSMS:PARAT MINI: Actual Study Start Date : November 3, 2022: Estimated Primary Completion Date : December 31, 2023 Effects of open-label placebos in clinical trials: a ... - Nature Web16 feb. 2021 · Eleven trials were eligible for meta-analysis. These trials assessed effects of OLPs on back pain, cancer-related fatigue, attention deficit hyperactivity disorder, allergic rhinitis, major... End of Trial and Open-Label Extension (OLE) Frequently Asked ... Open Label Extension, or OLE, is a phase of a study that occurs after the randomized (blinded) portion of the trial is completed if a drug is found to have the potential for benefit. Eligible trial participants take the active form of the drug without placebo. OLE allows active drug to be given to all participants at the same time and to follow them over time.
Epidemiology and Clinical Research Design, Part 1: Study Types. Avoids the problem of loss to follow-up. A cross-sectional study conducted at the beginning of a cohort or clinical trial provides demographic and clinical characteristics at baseline. Weaknesses: Cannot estimate incidence (the proportion who develop a disease or condition over time). Open-Label Trial - an overview | ScienceDirect Topics Positive Open-Label Trials. Two different open-label trials examined carbamazepine in pediatric bipolar disorder. 37,37a The Joshi trial studied carbamazepine extended release up to 788±252 mg/d in pediatric bipolar spectrum and monitored changes in 27 participants. Two participants had to discontinue because of rash, and only 16 subjects ... Open-Label Extension Studies | SpringerLink The number of open-label extension studies being performed has increased enormously in recent years. Often it is difficult to differentiate between these extension studies and the double-blind, controlled studies that preceded them. If undertaken primarily to gather more patient-years of exposure to the new drug in order to understand and gain confidence in its safety profile, open-label ... External and internal validity of open label or … Web15 sept. 2011 · The simplified anticoagulant treatment in an open label trial may be less challenging for the patient and might increase patient retention. On the other hand, …
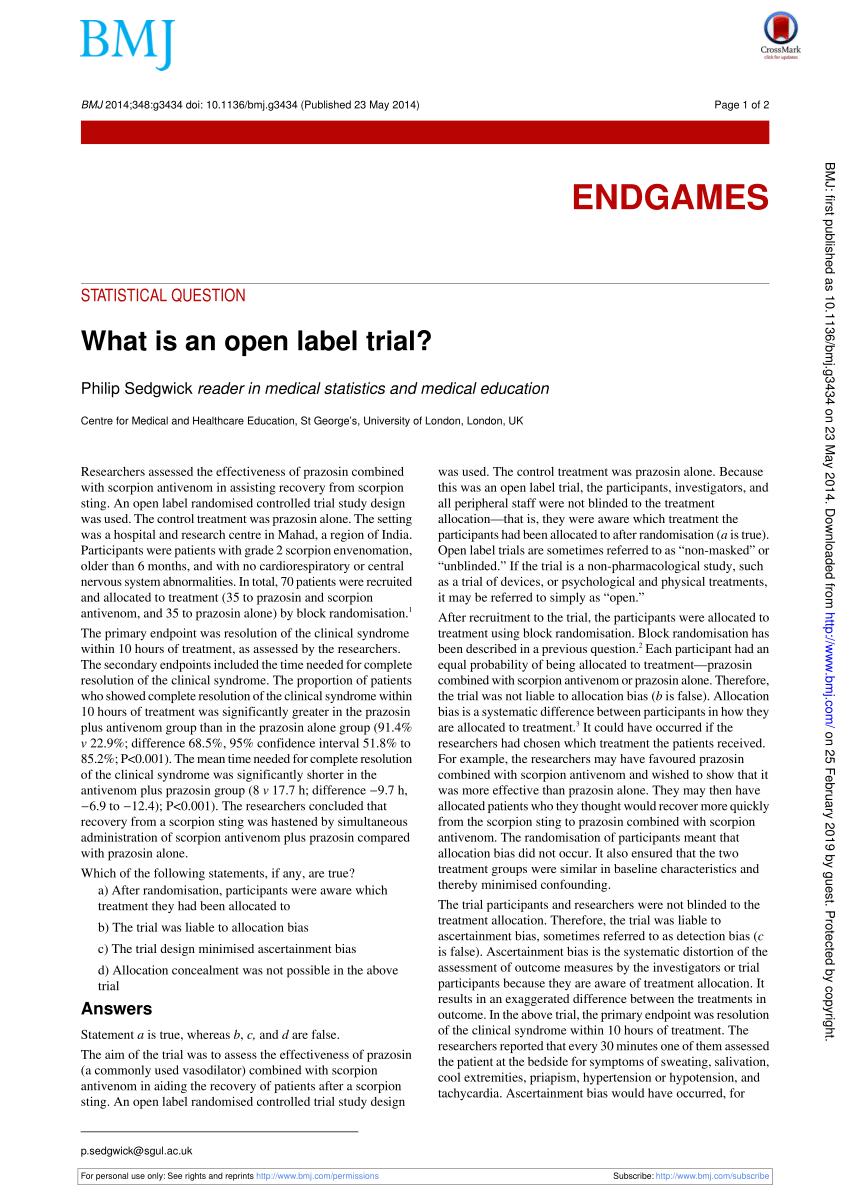
Open-label extension studies: do they provide meaningful … WebNegative aspects of open-label extension studies revolve around their use as a marketing tool, as they build a market for the drug and generate pressure for subsidised … Open-Label Trial | NIH - HIV.gov Open-Label Trial. A type of clinical trial. In open-label trials, both the researchers and participants know which drug (or other intervention) is being given to participants. Apa itu open-label trial? - Glosarium Online open-label trial : Percobaan klinis di mana dokter dan peserta percobaan itu mengetahui obat atau vaksin sedang diujicoba. Definisi ? semoga dapat membantu walau kurangnya jawaban pengertian lengkap untuk menyatakan artinya. pada postingan di atas pengertian dari kata "open-label trial" berasal dari beberapa sumber, bahasa, dan website di ... What is an open label trial? | The BMJ Web23 mai 2014 · Researchers assessed the effectiveness of prazosin combined with scorpion antivenom in assisting recovery from scorpion sting. An open label randomised …
Understanding Clinical Trial Terminology: What is an Open ... Jun 24, 2019 · Alternatively, sometimes, trials are conducted in an open-label fashion, meaning study participants and researchers both know which treatment the patient is receiving. Open-label trials can be used to compare treatments or gather additional information about the long-term effects in the intended patient population.
Effects of open-label placebos in clinical trials: a ... - Nature These trials assessed effects of OLPs on back pain, cancer-related fatigue, attention deficit hyperactivity disorder, allergic rhinitis, major depression, irritable bowel syndrome and menopausal...
Open-label extension studies: do they provide meaningful ... The number of open-label extension studies being performed has increased enormously in recent years. Often it is difficult to differentiate between these extension studies and the double-blind, controlled studies that preceded them. If undertaken primarily to gather more patient-years of exposure to …
Cancer trials are more likely to be open-label and not randomized More than 40,000 trials were registered between 2007 and 2010, with cancer trials representing 22 percent of those entered — the highest percentage of all clinical subspecialties. When compared with other specialties, cancer trials were more likely to be nonrandomized and open label.
Statistical controversies in clinical research: limitations of … WebOpen-label studies overestimated the risk of vascular adverse events with AA by at least 50%. Meta-analyses assessing adverse drug events should therefore be restricted to DB …
Impact of open-label versus blinded study design on … Web20 iul. 2021 · A high risk of attrition bias was detected in a higher proportion of open-label trials as compared to blinded trials (27% versus 13%), indicating that more open-label …
Treatment Use of Investigational Drugs | FDA Investigational products are sometimes used for treatment of serious or life-threatening conditions either for a single subject or for a group of subjects. The procedures that have evolved for an ...
External and internal validity of open label or double-blind trials in ... to the trial design of open-label vs. double-blind double-dummy (Table 1). For instance, while dabigatran was tested in AF using an open-label study [1] and in acute deep vein thrombosis (DVT) and pulmonary embolism (PE) using a double-blind double-dummy trial [2], rivaroxaban was tested inversely with open-label trials in acute DVT and PE [3 ...
External and internal validity of open label or double … WebIn general, a blinded trial is regarded as being less subject to bias than an open trial because it minimizes the impact of knowledge of treatment allocation on post …
What is an open label trial? | The BMJ May 23, 2014 · An open label randomised controlled trial study design was used. The control treatment was prazosin alone. The setting was a hospital and research centre in Mahad, a region of India. Participants were patients with grade 2 scorpion envenomation, older than 6 months, and with no cardiorespiratory or central nervous system abnormalities.
Bias for Patient-Reported Outcomes in Open-Label Cancer Trials: How Big ... A common concern with patient-reported outcomes (PROs) in open-label trials is that a patient's knowledge of treatment received could influence their view and reporting of their symptoms. With this in mind, members of the US Food and Drug Administration explored the possibility of such bias in a recent viewpoint published in JAMA Oncology.
Safety and Immunogenicity of a DNA SARS-CoV-2 ... - eClinicalMedicine First, this open-label, non-randomized Phase 1 trial report is based on a modest sample size (48) in all vaccine arms and, therefore lacks a comparator group. Larger sample-sized randomized placebo controlled blinded trials may be needed to show the true immunogenicity difference between the dose groups. Second, this report only involves ...
Open-Label Trial - an overview | ScienceDirect Topics The first mGluR5 antagonist tested in humans is the nonbenzodiazepine anxiolytic drug, fenobam. An open-label single-dose trial evaluated safety, pharmacokinetics, sensory gating, attention, impulsivity, and inhibition in 12 adult males and females. The single dose of fenobam was associated with a significant improvement of PPI compared to the ...
Reducing bias in open-label trials where blinded outcome … Web21 nov. 2014 · Many trial designs do not permit blinding, and are therefore designed as open-label, with patients, clinicians, and other study investigators aware of treatment …
Open-label trial - Wikipedia Open-label trials may be appropriate for comparing two similar treatments to determine which is most effective, such as a comparison of different prescription anticoagulants, [4] or possible relief from symptoms of some disorders when a placebo is given. [5] An open-label trial may still be randomized.
What is an Open-Label Clinical Trial? - News-Medical.net Clinical trials are vital for the design and development of safe drugs, treatments, and medical interventions and bringing them to market. Often, the idea for a clinical trial starts in the laboratory. Researchers will test new drugs and treatments in animal models, with the most promising being considered for clinical trials. There are four phases...
Open-label trial - Wikipedia WebOpen-label trials may be appropriate for comparing two similar treatments to determine which is most effective, such as a comparison of different prescription anticoagulants, or possible relief from symptoms of some disorders when a placebo is given. An open-label …






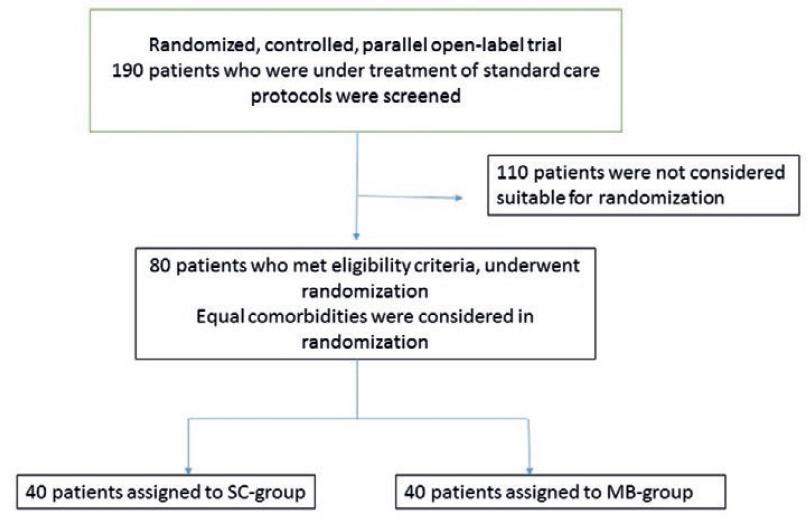



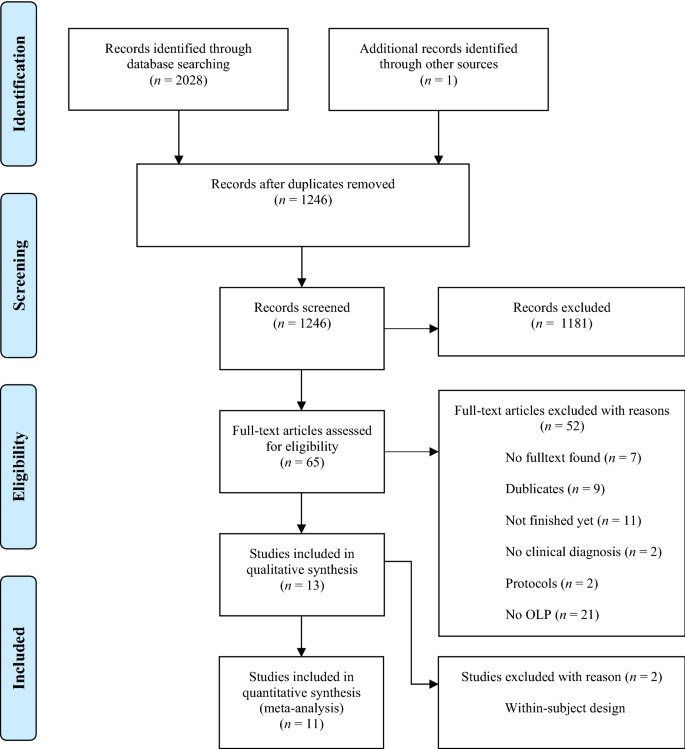

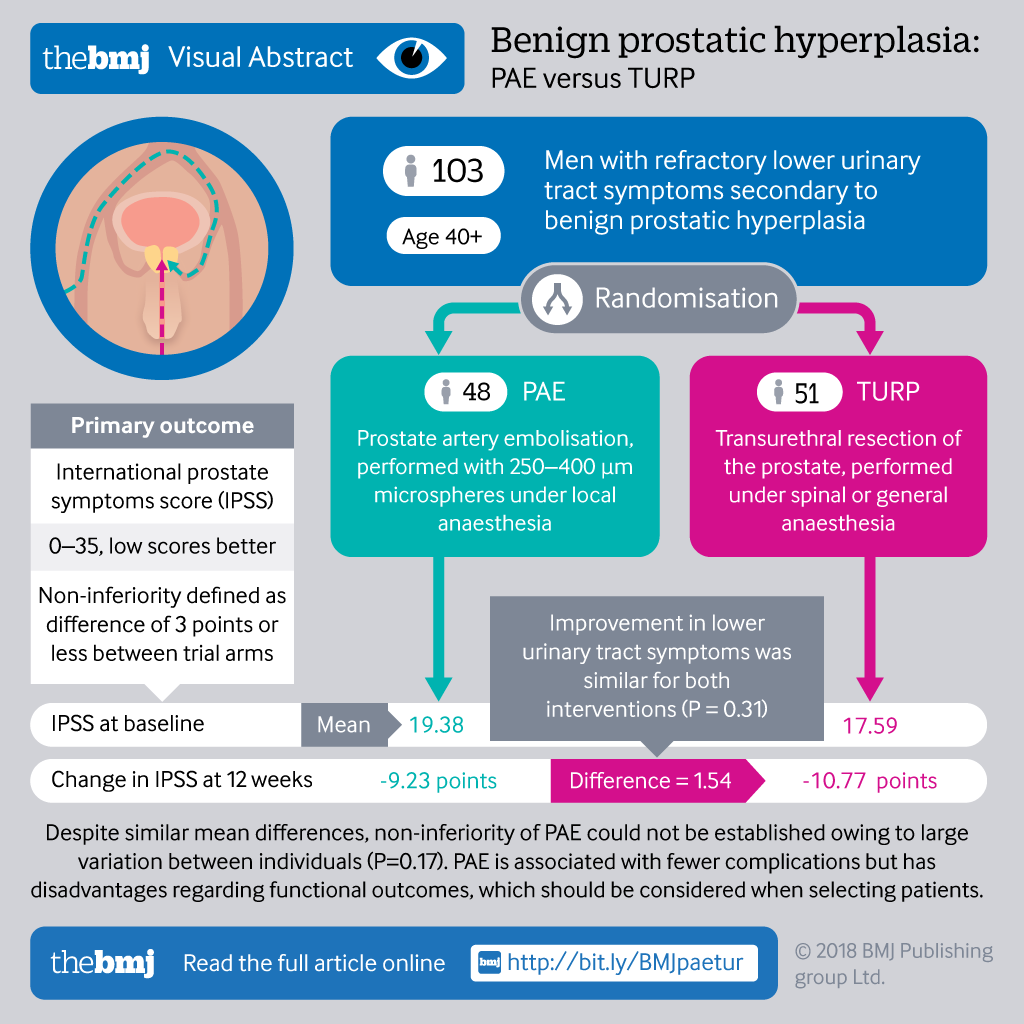




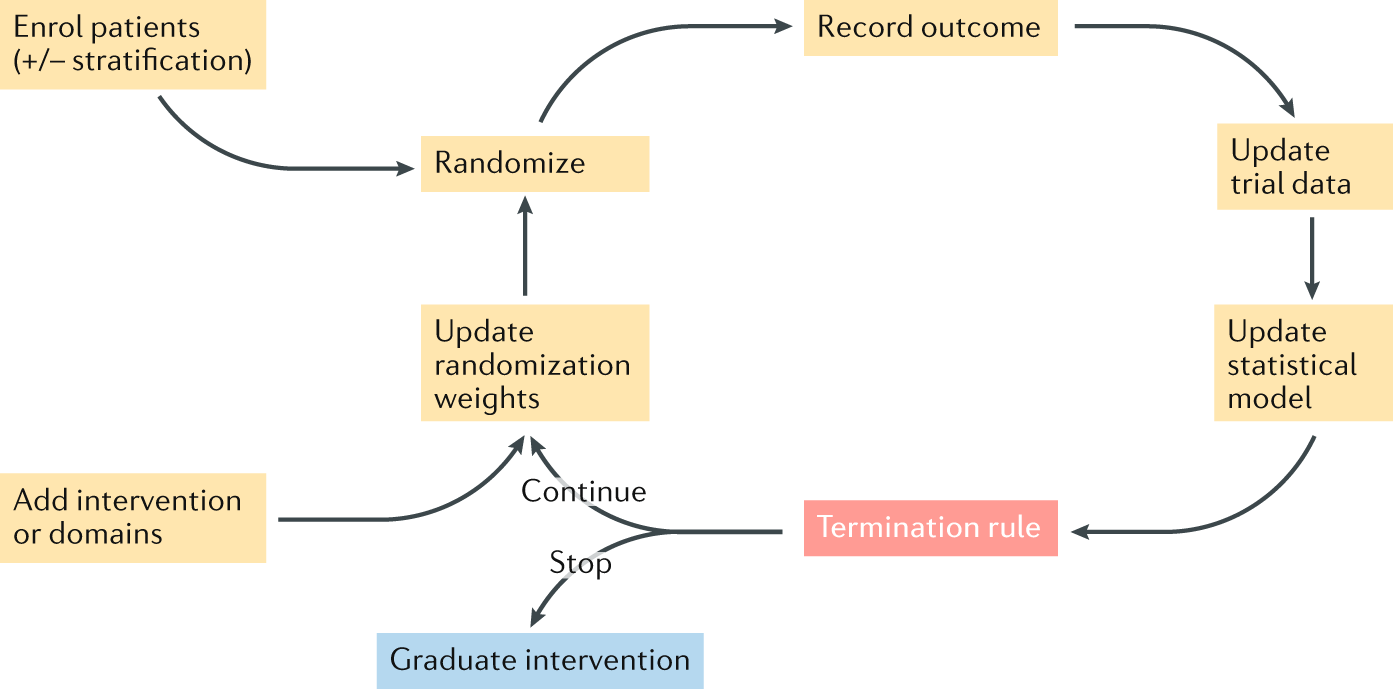



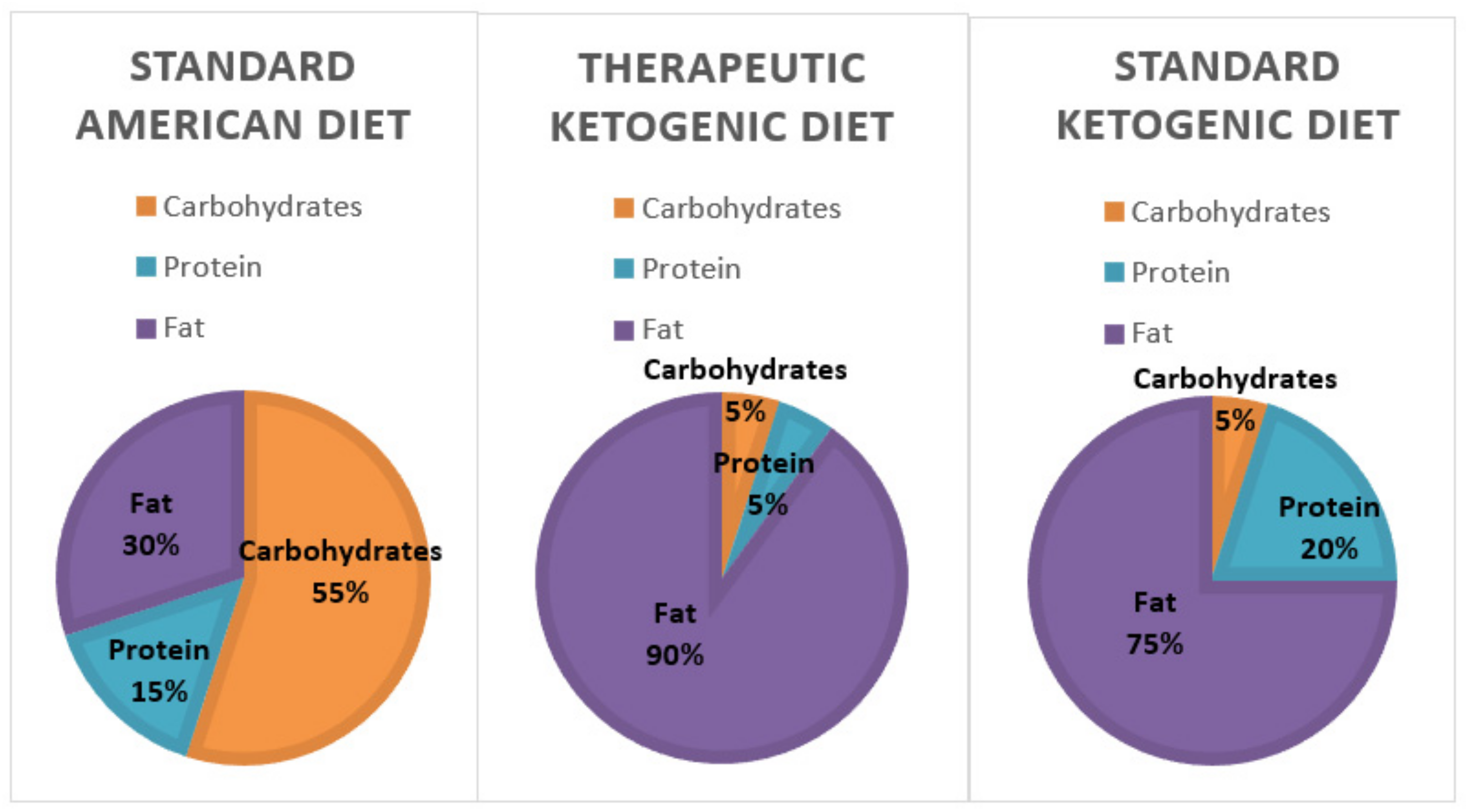




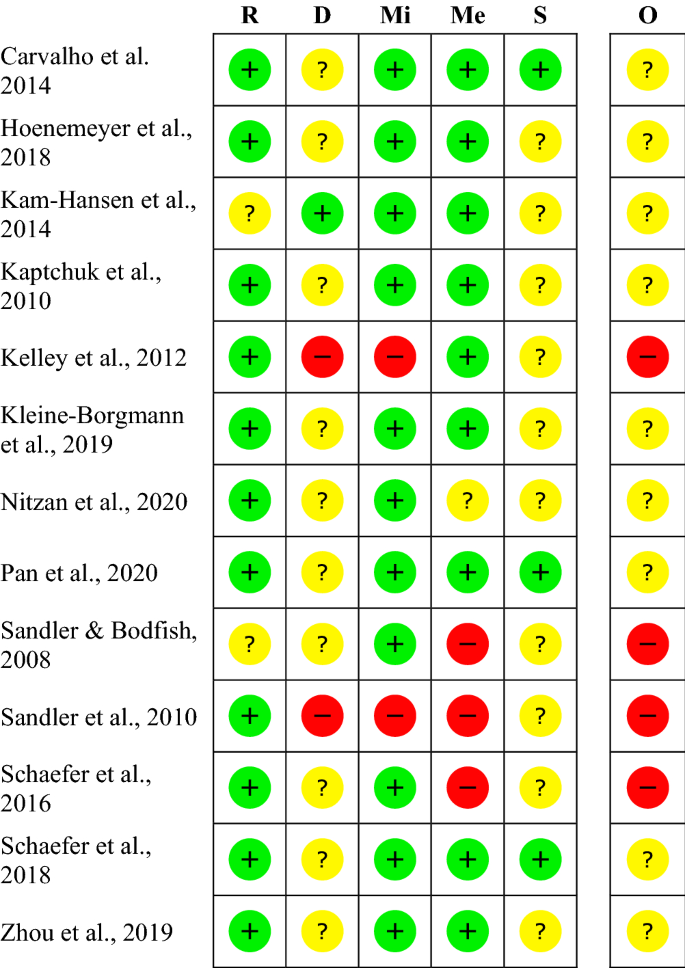


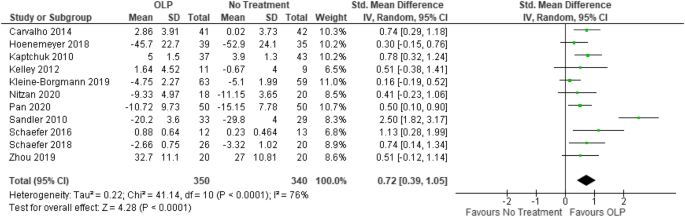

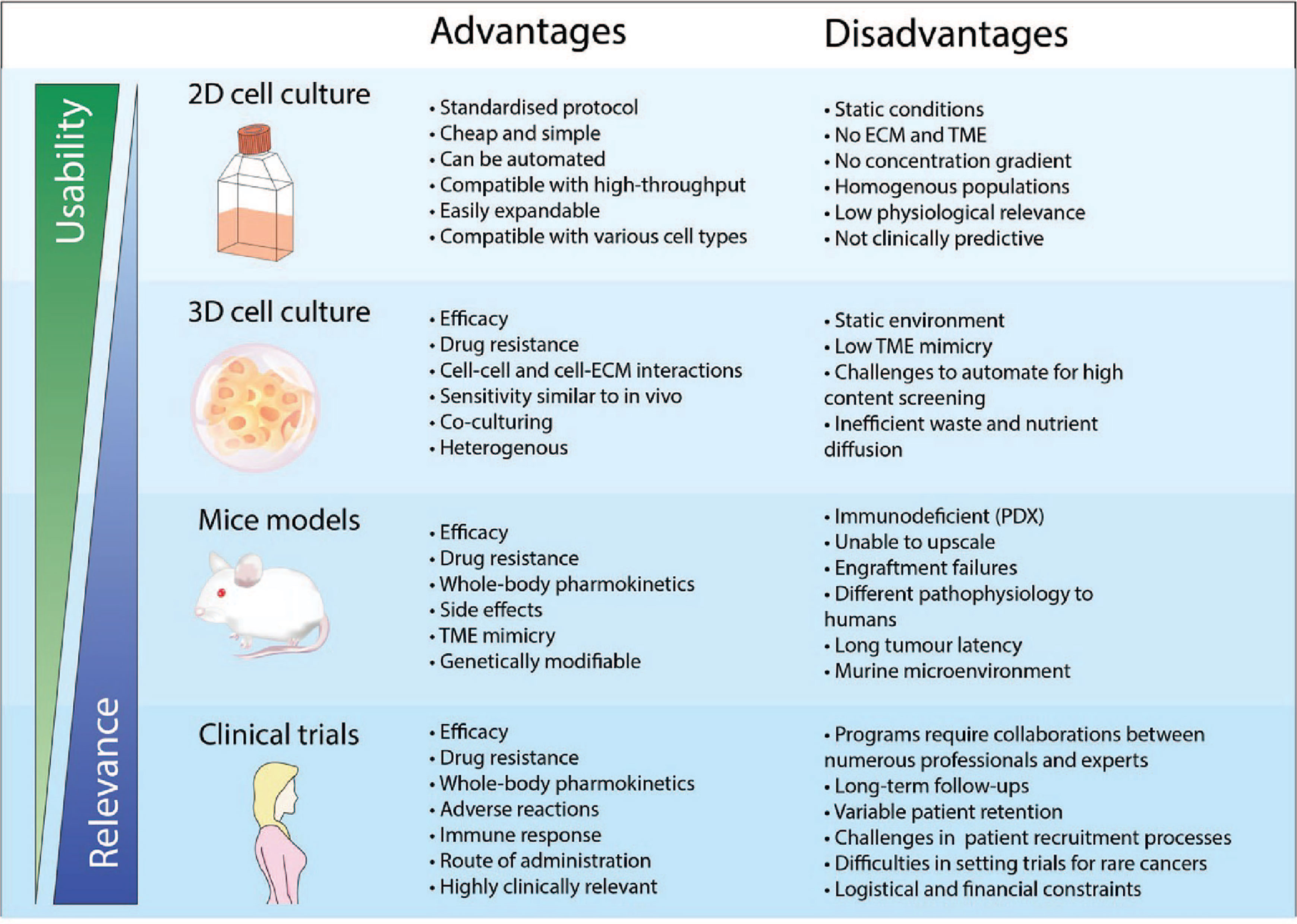


Post a Comment for "41 open-label trial disadvantages"