39 ph scale labelled
pH Scale | U.S. Geological Survey - USGS.gov The range goes from 0 - 14, with 7 being neutral. pHs of less than 7 indicate acidity, whereas a pH of greater than 7 indicates a base. pH is really a measure of the relative amount of free hydrogen and hydroxyl ions in the water. Water that has more free hydrogen ions is acidic, whereas water that has more free hydroxyl ions is basic. pH Scale | U.S. Geological Survey - USGS.gov An official website of the United States government. Here's how you know
pH Scale - Elmhurst University The pH scale ranges from 0 to 14. A pH of 7 is neutral. A pH less than 7 is acidic. A pH greater than 7 is basic. The pH scale is logarithmic and as a result, each whole pH value below 7 is ten times more acidic than the next higher value. For example, pH 4 is ten times more acidic than pH 5 and 100 times (10 times 10) more acidic than pH 6.
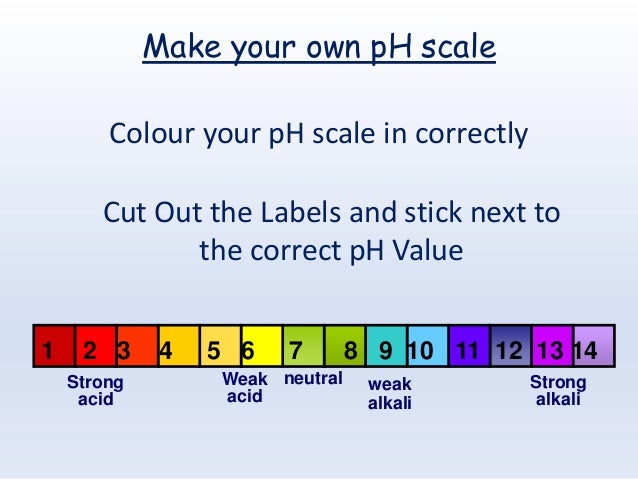
Ph scale labelled
Wender Utah Rating Scale-25 (WURS-25): psychometric … 29/10/2018 · The Wender Utah Rating Scale (WURS) is a self-report instrument that is designed to retrospectively assess childhood ADHD symptoms, based on the Utah criteria (10,11). The scale originally consisted of 61 items. The long form was arbitrarily reduced to the 25 items that showed the greatest mean difference between patients with ADHD and controls. In the original … The pH scale - Acids, bases and salts - (CCEA) - BBC Bitesize The pH scale measures a solution's acidity or alkalinity. The range for the pH scale is 0 (strong acid) to 14 (strong alkali). pH 0 - 2: strong acid pH 3 - 6: weak acid pH 7: neutral pH 8 ... pH Scale - Acids and Bases A pH of 7 is neutral on the scale, greater than 7 is a base and less than 7 is an acid. Strong acids are mostly ranged at a pH of 0-2, strong bases have a range at a pH of 12-14. Colour Indicators. Colour Indicators are used to determine how acidic, basic or neutral the solution is. This method is told by the changing colour of the substance ...
Ph scale labelled. Dissociative Experiences Scale - CounsellingResource.com 25/04/2011 · The left of the scale, labelled ‘Never’, corresponds to 0% of the time, while the right of the scale, labelled ‘Always’, corresponds to 100% of the time; the range covers 0% to 100% in 10% increments. Take the Quiz. Please note: This test will only be scored correctly if you answer each one of the questions. Draw neat and labeled diagram of pH scale? - Toppr The neat and labeled diagram of pH scale is as shown. The range of pH is from 0 to 14. pH 7 corresponds to neutral pH. pH less than 7 corresponds to acidic pH. pH more than 7 corresponds to alkaline pH. Was this answer helpful? 0 0 Diagram of the pH scale with examples of acidic, neutral and ... Download this stock image: Diagram of the pH scale with examples of acidic, neutral and alkaline substances. - G156N5 from Alamy's library of millions of ... pH Chemistry (Acids & Bases) - Definition, Calculating pH Value, Videos ... A pH scale is a tool for measuring acids and bases. The scale ranges from 0-14: Litmus paper is an indicator used to tell if a substance is an acid or a base. The colour of the paper matches up with the numbers on the pH scale to indicate what kind of substance is being tested. For example, Vinegar is an acid and measures 2.4 on the pH scale.
Wender Utah Rating Scale-25 (WURS-25): psychometric ... Oct 29, 2018 · The Wender Utah Rating Scale (WURS) is a self-report instrument that is designed to retrospectively assess childhood ADHD symptoms, based on the Utah criteria (10,11). The scale originally consisted of 61 items. The long form was arbitrarily reduced to the 25 items that showed the greatest mean difference between patients with ADHD and controls. The pH scale - BBC Bitesize The pH scale is a number scale from 0 to 14. It tells us how acidic or alkaline an aqueous solution is. The pH scale is used to classify as acidic, alkaline or neutral. Neutral solutions are... pH Scale: Acids, bases, pH and buffers (article) | Khan Academy The pH scale is often said to range from 0 to 14, and most solutions do fall within this range, although it's possible to get a pH below 0 or above 14. Anything below 7.0 is acidic, and anything above 7.0 is alkaline, or basic. Image modified from " Water: Figure 7 ," by OpenStax College, Biology, CC BY 4.0. Modification of work by Edward Stevens. Acids & Bases - pH Scale - Labelled diagram - Wordwall Acids & Bases - pH Scale - Labelled diagram Strong Acid, Moderate Acid, Weak Acid, Neutral, Weak Base, Moderate Base, Strong Base, pH 0-2, pH 3-4, pH 5-6, pH 7, pH 8-9, pH 10-11, pH 12-14.
The pH scale with some common examples The pH scale, with examples of common solutions and their pH values. Download/View For commercial use please contact us A GUIDE TO pH MEASUREMENT - Mettler Toledo Thanks to accurate pH control it is possible to: manufacture a product with defined attributes produce a product at low cost prevent damage to the environment, materials and humans satisfy legal regulations gain further knowledge in research Table 1: pH scale range pH H+ concentration (mol/L) OH-concentration (mol/L) 0 1 0,00000000000001 Making a pH indicator using red cabbage - RSC Education A pH indicator is a substance which has one colour when added to an acidic solution and a different colour when added to an alkaline solution. Various colouring materials in plants can act as indicators. In this practical, students make an indicator from red cabbage. The experiment is in two parts. In the first part, students boil some red ... The pH Scale | Biology for Non-Majors I | | Course Hero The pH scale, which measures from 0 to 14, provides an indication of just how acidic or basic a substance is. Most parts of our body (excluding things like stomach acid) measure around 7.2 and 7.6 on the pH scale (a 7 is neutral on the scale). If foreign strong substances dramatically change this pH, our bodies can no longer function properly.
BYJUS BYJUS
VISTA is an acidic pH-selective ligand for PSGL-1 | Nature Oct 23, 2019 · b, Human monocytes (left) and neutrophils (right) labelled with VISTA multimers at pH 6.0 and pH 7.4. Cells labelled with non-VISTA-loaded multimers (control) or left unstained (FMO) are also ...
pH Scale - Labelled diagram - Wordwall Neutral, Weak Acid, Strong Acid, Weak Alkali, Strong Alkali, Stomach Acid, Water, Indigestion Tablets, Drain Cleaner.
What is the pH Scale? - ReAgent Chemical Services The pH scale is a precise way of classifying the acidity, basicity or neutrality of a solution. As a logarithmic scale, 1 pH unit is ten times stronger, or ten times weaker, than the one below or above it, depending on its position: a pH of 4 is ten times more acidic than a pH of 5, but ten times weaker than a pH of 3.
arXiv:2205.09091v1 [astro-ph.IM] 18 May 2022 19/05/2022 · be sensitive to changes or features on an angular scale of ˘ =Bfrom the source, where Bis the baseline sep-aration between the apertures and is the photon wave- length. The Michelson setup necessitates maintaining a stable optical path between the stations, which typically limits practical baselines of optical interferometers to a few 100s of meters [1{3]. This …
pH - Wikipedia In chemistry, pH (/ p iː ˈ eɪ tʃ /), historically denoting "potential of hydrogen" (or "power of hydrogen") is a scale used to specify the acidity or basicity of an aqueous solution.Acidic solutions (solutions with higher concentrations of H + ions) are measured to have lower pH values than basic or alkaline solutions.. The pH scale is logarithmic and inversely indicates the …
Several substances are labeled on the pH scale below. A p H scale is ... Several substances are labeled on the pH scale below. A p H scale is shown ranging from 0 the top to 14 at the bottom. The approximate locations of substances are: gastric fluid, 1.2; lemon juice, 2.2; milk, 6.5; blood, 7.5; seawater, 8; milk of magnesia, upper M g (upper O upper H) subscript 2 solution, 10.5; household lye, upper N a upper O upper H solution, 13.5.
Dissociative Experiences Scale - CounsellingResource.com Apr 25, 2011 · The left of the scale, labelled ‘Never’, corresponds to 0% of the time, while the right of the scale, labelled ‘Always’, corresponds to 100% of the time; the range covers 0% to 100% in 10% increments. Take the Quiz. Please note: This test will only be scored correctly if you answer each one of the questions.
Acids, Alkalis, and the pH Scale - Compound Interest A pH of spot on 7 denotes a neutral solution (neither acidic or alkaline). Any pH below 7 is acidic, whilst any pH above 7 is termed alkaline. Water molecules have the chemical formula H 2 O. However, these molecules are capable of splitting up slightly in solution, in H + and OH - (hydroxide) ions.




Post a Comment for "39 ph scale labelled"