45 what is used to label the energy levels of electrons
Isotope - Wikipedia Isotope vs. nuclide. A nuclide is a species of an atom with a specific number of protons and neutrons in the nucleus, for example carbon-13 with 6 protons and 7 neutrons. The nuclide concept (referring to individual nuclear species) emphasizes nuclear properties over chemical properties, whereas the isotope concept (grouping all atoms of each element) emphasizes … Properties of Periodic Table of Element Groups - ThoughtCo Apr 01, 2016 · Electronegativity and ionization energy intermediate between that of metals and nonmetals; May possess a metallic luster; Variable density, hardness, conductivity, and other properties; Often make good semiconductors; Reactivity depends on …
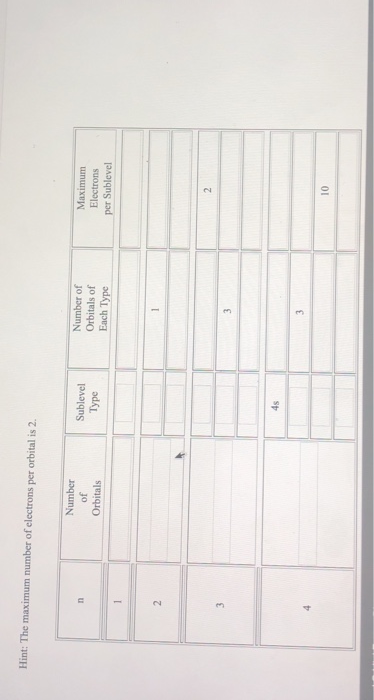
Chapter 5 test Section 5.1 Flashcards - Quizlet The term that is used to label the energy levels of electrons is? S What letter is used to denote a spherical orbital? Dumbell All "p" orbital's are _____ shaped? 2n^2 What is the formula for the maximum number of electrons that can occupy a principal energy level? (Use "n" for the principal quantum number) Recommended textbook explanations
What is used to label the energy levels of electrons
ATP synthesis and storage - PMC Apr 12, 2012 · In 1964, Daniel Atkinson proposed the energy-charge hypothesis, which stated that regulatory enzymes involved in fundamental pathways for a correct development and survival of the cell, would be sensitive to the energy charge, that is, to ATP levels. To confirm this hypothesis, a series of studies on enzymes was conducted. 8.8: Term Symbols Gives a Detailed Description of an Electron ... Mar 18, 2020 · The problem with this idea is that the angular momenta of the various electrons are not necessarily pointing in the same direction. If two electrons are revolving in the same direction as each other, you would add their \(l\) values. \[ L = \sum_i^n l_i \label{8.8.4A}\] If they were revolving opposite to each other, you would subtract them. 5: Quantum Energy Levels in Atoms - Chemistry LibreTexts Careful observation and analysis reveals that every frequency in the hydrogen atom spectrum can be predicted by a very simple formula, called the Rydberg equation: (5.2) ν = R × ( 1 n 2 − 1 m 2) where R is the Rydberg constant ( 3.29 × 10 15 s − 1). n and m are integers (1,2,3,...). Each choice of n and m predicts a single observed ...
What is used to label the energy levels of electrons. Energy Level of an Atom - Energy State and Energy level Diagrams - VEDANTU Energy level diagrams are the representation of placements or arrangements of orbitals (also known as subshells) according to their increasing energy levels. Above is the blank energy level diagram which can be used to represent the electrons for any atom under study. Energy level diagrams are known as Grotrian diagrams. Energy Level - Principal Quantum Number | Bohr's Atomic Model | Physics These stationary states/ energy level for an electron are numbered as n = 1, 2, 3……….. These integers are also known as the principal quantum numbers. Energy of the stationary state in which an electron is placed is given by: Where, R H is called Rydberg constant whose value is 2.18×10 -18 J. ATOM, ORBITS AND ENERGY LEVELS - PIJA Education Orbits (shells) are the energy levels of atoms in which electrons move around the nucleus. Energy of electrons in 1 st orbit is less than energy of electrons in 2 nd orbit. Similarly, the energy of electrons in 2 nd orbit is less than that of 3 nd orbit. In other words, energy of electrons in inner orbits has less energy compared to outer orbits. 2.2: Atomic Orbitals and Quantum Numbers Jun 5, 2019 — The energy levels are labeled with an n value, where n = 1, 2, 3, …. Generally speaking, the energy of an electron in an atom is greater for ...
6.2: Semiconductors and Energy Level Diagrams Energy is on the vertical axis. Allowed energy levels are shown by horizontal lines. Each electron can only have energy corresponding to one of these discrete possible energy levels. At \(T = 0\) K, electrons occupy the lowest possible energy levels. One electron can occupy each line, so the lowest 13 energy levels are occupied by electrons. Term Symbols for Atomic Energy Levels - Georgia State University Term Symbols for Atomic Energy Levels Term Symbols The heirarchy of labels for the electrons of multi-electron atoms is configuration, term, level, and state. The term uses the multiplicity 2S + 1, total orbital angular momentum L, and total angular momentum J. Energy level - Wikipedia A quantum mechanical system or particle that is bound—that is, confined spatially—can only take on certain discrete values of energy, called energy levels. This contrasts with classical particles, which can have any amount of energy. The term is commonly used for the energy levels of the electrons in atoms, ions, or molecules, which are bound by the electric field of the nucleus, but can also refer to energy levels of nuclei or vibrational or rotational energy levels in molecules. The ... What term is used to label the energy levels of electrons? - Answers What term is used to label energy levels of electrons? Principal quantum numbers (n). What is the term valance band? It refers to the energy levels in an atom where the electrons that participate...
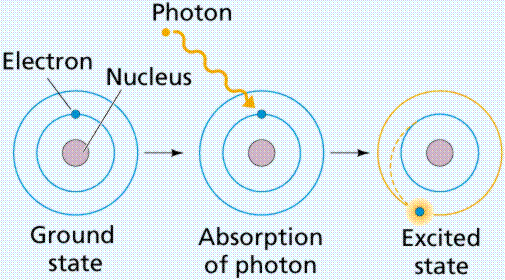
s,p,d,f Orbitals - Chemistry | Socratic An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball made of rather fluffy material with the nucleus at its centre. As the energy levels increase, the electrons are located further from the nucleus, so the orbitals get bigger. The order of size is 1s < 2s < 3s < …, as shown below. Define the following: a. main energy levels b. quantum num Main energy levels, or shell, represents a fixed distances from the nucleus where we can find electrons. It is symbolized by 'n', and as the value of 'n' ... Electron Energy Level - an overview | ScienceDirect Topics Photoelectron spectroscopy is a technique whereby electrons directly ejected from the surface region of a solid by incident photons are energy analyzed and the spectrum is then related to the electron energy levels of the system. Energy Level and Transition of Electrons - Brilliant According to Bohr's theory, electrons of an atom revolve around the nucleus on certain orbits, or electron shells. Each orbit has its specific energy level, which is expressed as a negative value. This is because the electrons on the orbit are "captured" by the nucleus via electrostatic forces, and impedes the freedom of the electron.
Circuit Construction Kit: DC - Series Circuit | Parallel Circuit - PhET Experiment with an electronics kit! Build circuits with batteries, resistors, ideal and non-Ohmic light bulbs, fuses, and switches. Determine if everyday objects are conductors or insulators, and take measurements with an ammeter and voltmeter. View the circuit as a schematic diagram, or switch to a lifelike view.
Intro to Electricity - New York University •A resistor is a dissipative element. It converts electrical energy into heat energy. It is analogous to the viscous friction element of mechanical system. •When electrons enter at one end of a resistor, some of the electrons collide with atoms within the resistor. These atoms start vibrating and transfer their energy to neighboring air ...
Ventilation (architecture) - Wikipedia Ventilation guidelines are based upon the minimum ventilation rate required to maintain acceptable levels of bioeffluents. Carbon dioxide is used as a reference point, as it is the gas of highest emission at a relatively constant value of 0.005 L/s. The mass balance equation is: Q = G/(C i − C a) Q = ventilation rate (L/s) G = CO 2 generation ...
Solved An orbital energy diagram is used to show the order - Chegg Question: An orbital energy diagram is used to show the order in which electrons are assigned to energy levels. For the diagram below, label each energy level with the correct n quantum number and the correct subshell designation, s, p ord. This problem has been solved! See the answer Show transcribed image text Expert Answer 92% (50 ratings)
PDF Review of energy levels (atomic orbitals) - Cal State LA The energy levels for electrons in atoms are called atomic orbitals: 1. Quantized (discrete) energy levels. 2. The electron is a three-dimensional wave-particle that is delocalized over space without an exact location or exact motion. Fig. 7.10, Figs. 7.13-7.15. 3. The periodic table lists atomic orbitals in order from lowest to highest energy ...
What is the term used to label the energy levels of electron Answer: The quantum mechanical model of the atom estimates the probability of finding an electron in a certain position. ... Circle the letter of the formula for the maximum number of electrons that can accupy a principal energy level. Use n for the principal quantum number. Advertisement Previous Next
How to Represent Electrons in an Energy Level Diagram Chemists use the energy level diagram as well as electron configuration notation to represent which energy level, subshell, and orbital are occupied by electrons in any particular atom. Chemists use this information in these ways: To predict what type of bonding will occur with a particular element and show exactly which electrons are being used
Energy Levels and Electron Configurations - Periodic Trends As we've said, each orbital has a specific energy. To be more specific, in a multi-electron atom, energy increases as the value (n+ l) increases. For orbitals with the same (n+ l) values, the lower energy orbitals have the lower n values. This means that orbitals with the same n increase in energy as l increases - so the 2s orbital ( l =0) is ...



Post a Comment for "45 what is used to label the energy levels of electrons"