39 open label meaning
dom-pubs.onlinelibrary.wiley.com › doi › 10Long‐term safety and efficacy of imeglimin as monotherapy or ... Dec 06, 2021 · Of the 981 patients who provided signed informed consent, 714 entered the open-label treatment period and received at least one dose of imeglimin, including 134 patients with imeglimin long-term monotherapy, and 580 patients with imeglimin long-term combination therapy: 64 patients with an AGI, 64 patients with a BIG, 63 patients with a DPP4-I ... Open label extension studies: research or marketing? 10.09.2005 · Open label extension studies typically follow a double blind randomised placebo controlled trial of a new drug. At the end of the double blind phase, participants are invited to enrol in an extension study. The study will normally be longer than the randomised trial (two years is not uncommon but they often continue until the drug is licensed). All participants in the …
Open-label - definition of open-label by The Free Dictionary Define open-label. open-label synonyms, open-label pronunciation, open-label translation, English dictionary definition of open-label. adj. Of or related to a clinical trial in which both the researcher and the participant know whether an administered treatment is an …
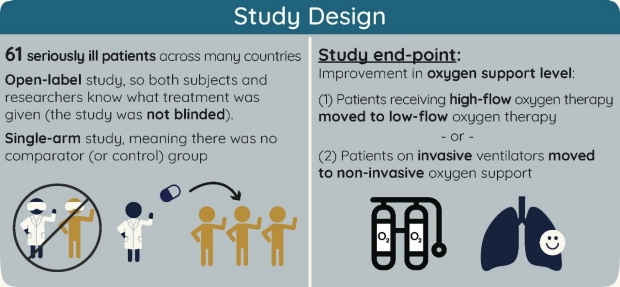
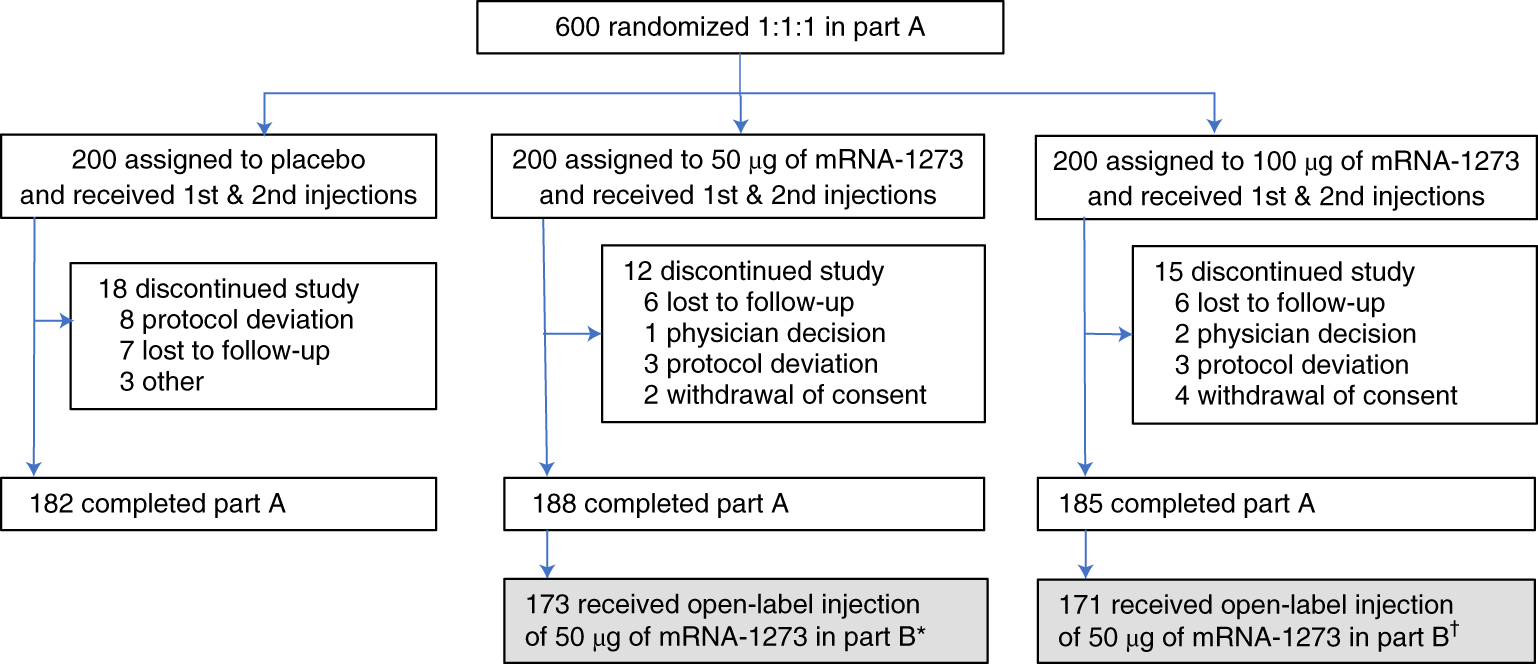
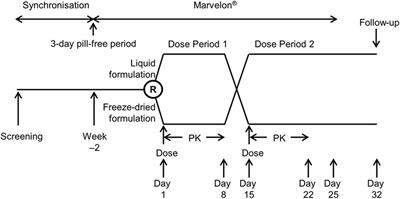
Open label meaning
(PDF) What is an open label trial? - ResearchGate An open label randomised controlled trial study design was used. ... The mean time needed for complete resolution of the clinical syndrome was significantly shorter in the antivenom plus prazosin ... What is an open label extension study? • NCK Pharma An open - label trial or open trial is a type of clinical trial in which both the researchers and participants know which treatment is being administered. NCI Dictionary of Cancer Terms NCI's Dictionary of Cancer Terms provides easy-to-understand definitions for words and phrases related to cancer and medicine.
Open label meaning. Open-label extension studies: do they provide meaningful information on ... Open-label extension studies do have a legitimate but limited place in the clinical development of new medicines. The negative perceptions about these studies have arisen because of perversion of acceptable rationales for this type of study and a failure to recognise (or disclose) the limitations resulting from the inherent weaknesses in their ... › daisy-flower-meaningDaisy Flower Meaning - Flower Meaning Wild daisy tea is said to be good for ailments of the throat, for putting on open wounds and as a “blood purifier” (whatever that means) but there aren’t any clinical studies to back up these traditional claims. People who are allergic to ragweed are very likely to also be allergic to daisies or any products made from daisies. Medical Definition of Open-label trial - RxList Definition of Open-label trial. Medical Editor: Melissa Conrad Stöppler, MD; Reviewed on 3/29/2021. open-label_trial Open-label trial: A clinical trial in which researchers and participants know which drug or vaccine is being administered. From . Featured Centers. Good and Bad Foods for Psoriasis ... Open-label Definition & Meaning - Merriam-Webster Definition of open-label : being or relating to a clinical trial in which the treatment given to each subject is not concealed from either the researchers or the subject an open-label multicenter study — compare double-blind, single-blind First Known Use of open-label 1979, in the meaning defined above Learn More About open-label
Medical Definition of Open-label trial - MedicineNet medterms medical dictionary a-z list / open-label trial definition Medical Definition of Open-label trial. Medical Editor: Melissa Conrad Stöppler, MD; Reviewed on 3/29/2021. Open-label trial: A clinical trial in which researchers and participants know which drug or vaccine is being administered. CONTINUE ... Label Definition & Meaning - Merriam-Webster label: [verb] to affix a label to. to describe or designate with or as if with a label. What is an open label trial? | The BMJ An open label randomised controlled trial study design was used. The control treatment was prazosin alone. The setting was a hospital and research centre in Mahad, a region of India. Participants were patients with grade 2 scorpion envenomation, older than 6 months, and with no cardiorespiratory or central nervous system abnormalities. › journals › lanoncUpfront FOLFOXIRI plus bevacizumab with or without ... May 27, 2022 · AtezoTRIBE was a multicentre, open-label, randomised, controlled, phase 2 study of patients (aged 18–70 years with an Eastern Cooperative Oncology Group [ECOG] performance status of 0–2 and aged 71–75 years with an ECOG performance status of 0) with histologically confirmed, unresectable, previously untreated metastatic colorectal cancer and adequate organ function, who were recruited ...
Medical Definition of Open-label - MedicineNet Open-label: A term used to describe the situation when both the researcher and the participant in a research study know the treatment the participant is receiving. Open-label is the opposite of double-blind when neither the researcher nor the participant knows what treatment the participant is receiving. CONTINUE SCROLLING OR CLICK HERE QUESTION FDA-Approved Label vs. Off-Label: What Does It Mean? Botox is FDA approved for the crows feet and glabella area (the area between the eyes) to reduce the appearance of fine lines and wrinkles. However, Botox is often used in the forehead, nose, lip, jaw, chin, and underarm areas, as well, meaning these are "off-label" uses for the prescription. Likewise, some fillers, such as Juvederm, Voluma ... PDF Blinding Sponsors for Open Label Studies: Challenges and Solutions - MWSUG In open label studies, in addition to treatment assignment data itself, many other data elements in the CRF data can reveal treatment assignment. Those data elements include (but not limited to): • A common variable that is collected for all treatment arms but takes different values for different treatment arms. What is an open label trial? | The BMJ 23.05.2014 · An open label randomised controlled trial study design was used. The control treatment was prazosin alone. The setting was a hospital and research centre in Mahad, a region of India. Participants were patients with grade 2 scorpion envenomation, older than 6 months, and with no cardiorespiratory or central nervous system abnormalities. In total, 70 …
Medical Definition of Open-label - MedicineNet 29.03.2021 · Open-label: A term used to describe the situation when both the researcher and the participant in a research study know the treatment the participant is receiving. Open-label is the opposite of double-blind when neither the researcher nor the participant knows what treatment the participant is receiving. CONTINUE SCROLLING OR CLICK HERE.
en.wikipedia.org › wiki › Interval_(mathematics)Interval (mathematics) - Wikipedia In many contexts, an -dimensional interval is defined as a subset of that is the Cartesian product of intervals, =, one on each coordinate axis.. For =, this can be thought of as region bounded by a square or rectangle, whose sides are parallel to the coordinate axes, depending on whether the width of the intervals are the same or not; likewise, for =, this can be thought of as a region ...
Understanding Clinical Trial Terminology: What is an Open Label ... Alternatively, sometimes, trials are conducted in an open-label fashion, meaning study participants and researchers both know which treatment the patient is receiving. Open-label trials can be used to compare treatments or gather additional information about the long-term effects in the intended patient population.
Open-label trial | definition of open-label trial by Medical dictionary Open-label trial | definition of open-label trial by Medical dictionary open-label trial Also found in: Wikipedia . open-label study A clinical study in which the patients/subjects and investigators know which product each patient/subject is receiving, which is the opposite of a blinded study. Segen's Medical Dictionary. © 2012 Farlex, Inc.
Open-label trial - Wikipedia An open-label trial, or open trial, is a type of clinical trial in which information is not withheld from trial participants. In particular, both the researchers and participants know which treatment is being administered. This contrasts with a double-blinded trial, where information is withheld both from the researchers and the participants to ...
Open-label - definition of open-label by The Free Dictionary open-label Also found in: Medical, Wikipedia . o·pen-la·bel (ō′pən-lā′bəl) adj. Of or related to a clinical trial in which both the researcher and the participant know whether an administered treatment is an experimental drug or a placebo. American Heritage® Dictionary of the English Language, Fifth Edition.
Open-label study | definition of open-label study by Medical dictionary open-label study a study in which there is no blinding of treatments. Farlex Partner Medical Dictionary © Farlex 2012 open-label study A clinical study in which the patients/subjects and investigators know which product each patient/subject is receiving, which is the opposite of a blinded study. Segen's Medical Dictionary. © 2012 Farlex, Inc.
finance.yahoo.com › news › zynerba-pharmaceuticalsZynerba Pharmaceuticals Announces Positive Top Line Results ... Jun 22, 2022 · The 14-week INSPIRE trial was an open-label, Phase 2 clinical trial designed to evaluate the safety, tolerability and efficacy of Zygel in children and adolescents (ages four through 15) with ...
What does open-label mean? - definitions.net What does open-label mean? Definitions for open-label open-la·bel Here are all the possible meanings and translations of the word open-label.
open.umn.edu › opentextbooks › textbooksIntroduction to Art: Design, Context, and Meaning - Open ... May 08, 2022 · Introduction to Art: Design, Context, and Meaning offers a comprehensive introduction to the world of Art. Authored by four USG faculty members with advance degrees in the arts, this textbooks offers up-to-date original scholarship. It includes over 400 high-quality images illustrating the history of art, its technical applications, and its many uses.
Open-Label Trial | NIH Open-Label Trial Audio Your browser does not support the audio element. 530.mp3 A type of clinical trial. In open-label trials, both the researchers and participants know which drug (or other intervention) is being given to participants. Related Term(s) Clinical Trial Double-Blind Study Print Print this term Download Glossary
Meaning of "Open Label Pilot study"..?? - SAS Support Communities Open label is a term used to describe the situation when both the researcher and the participant in a research study know the treatment the participant is receiving. Open-label is the opposite of double-blind when neither the researcher nor the participant knows what treatment the participant is receiving.
Open-label extension studies: do they provide meaningful … Open-label extension studies do have a legitimate but limited place in the clinical development of new medicines. The negative perceptions about these studies have arisen because of perversion of acceptable rationales for this type of study and a failure to recognise (or disclose) the limitations resulting from the inherent weaknesses in their design. Increased …
(PDF) What is an open label trial? - ResearchGate 23.05.2014 · In addition, the open-label design of SaROD increases the chances of ascertainment bias compared with the doubleblind design used in OPERA I and II (Chan et al., 2013; Sedgwick, 2014). This is ...
Open-Label Trial - an overview | ScienceDirect Topics An open-label trial has no blinding: everyone knows which patient is receiving which treatment. Open-label studies lack the rigor of blinded studies. Since the lack of blinding can introduce significant bias, reserve the use of open-label studies for situations in which blinding is neither feasible nor ethical or in cases where the outcome is ...
Open-label, randomized, parallel-group controlled clinical trial of ... Objectives: The study objectives were to determine whether massage therapy reduces symptoms of depression in subjects with human immunodeficiency virus (HIV) disease. Design: Subjects were randomized non-blinded into one of three parallel groups to receive Swedish massage or to one of two control groups, touch or no intervention for eight weeks.
Understanding Clinical Trial Terminology: What is a Long-Term Extension ... Clinical trials are important research studies that are conducted to test potential new medicines in people after earlier stages of research have been successfully completed. Clinical trials typically move forward in a series from early-stage, small-scale Phase 1 studies to late-stage, large-scale, Phase 3 studies. These studies could be double-blind in which case the trial […]
Open-label trial - Wikipedia Open-label trial. An open-label trial, or open trial, is a type of clinical trial in which information is not withheld from trial participants. [1] In particular, both the researchers and participants know which treatment is being administered. [1] This contrasts with a double-blinded trial, where information is withheld both from the researchers ...
› journals › laneurTenecteplase versus alteplase for the management of acute ... May 04, 2022 · This phase 3, randomised, open-label, blinded endpoint, non-inferiority trial was performed at 11 hospitals with stroke units in Norway. Patients with suspected acute ischaemic stroke with a National Institutes of Health Stroke Scale score of 6 or more who were eligible for thrombolysis and admitted within 4·5 h of symptom onset were consecutively included.
End of Trial and Open-Label Extension (OLE) Frequently Asked Questions ... Open Label Extension, or OLE, is a phase of a study that occurs after the randomized (blinded) portion of the trial is completed if a drug is found to have the potential for benefit. Eligible trial participants take the active form of the drug without placebo. OLE allows active drug to be given to all participants at the same time and to follow them over time.
PDF What Are Open-Label Extension Studies For? factors. In the case of open-label extension studies, we feel that analysis of efficacy ought to regard treatment allocation as a possible explanatory variable (in addition to others, such as baseline status and time). The study by Mease and colleagues 1 reports the results of a 48-week open-label extension of a 24-week randomized
Lable vs. Label - Which is Correct? - School & Travel Meaning of Label: Basically, a label is a piece of material placed on a product for easy identification because it contains vital information about the product which includes where and who made it. On the other hand, a label can also be a name given to a category of products for proper identification. In the coding space, a label is a name ...
Open label extension studies: research or marketing? - PMC Open label extension studies are commonly used to assess long term tolerability of a new drug. However, a proportion of the participants eligible for the study will have already taken the study drug. Those who are unable to tolerate it are therefore unlikely to take part in the extension study.
Reducing bias in open-label trials where blinded outcome assessment is ... TAPPS was an open-label trial whose primary outcome was whether the patient was referred for a pleural drainage procedure. Allowing a blinded assessor to decide whether to refer the patient for a procedure was not feasible as many clinicians may be reluctant to enrol patients into the trial if they cannot be involved in their care during follow ...
Understanding Clinical Trial Terminology: What is an Open Label ... 24.06.2019 · Alternatively, sometimes, trials are conducted in an open-label fashion, meaning study participants and researchers both know which treatment the patient is receiving. Open-label trials can be used to compare treatments or gather additional information about the long-term effects in the intended patient population. In some instances, patients who complete …
NCI Dictionary of Cancer Terms NCI's Dictionary of Cancer Terms provides easy-to-understand definitions for words and phrases related to cancer and medicine.
What is an open label extension study? • NCK Pharma An open - label trial or open trial is a type of clinical trial in which both the researchers and participants know which treatment is being administered.
(PDF) What is an open label trial? - ResearchGate An open label randomised controlled trial study design was used. ... The mean time needed for complete resolution of the clinical syndrome was significantly shorter in the antivenom plus prazosin ...
























![PDF] Corporate governance and its contribution to risk and ...](https://d3i71xaburhd42.cloudfront.net/b41d6158254ff706ff0b4f1abe1b906bcb441399/239-Table33-1.png)

Post a Comment for "39 open label meaning"